Enzyme-linked immunosorbent assay (ELISA) is a cornerstone of laboratory research, renowned for its ability to detect and quantify proteins, antibodies, and hormones. When paired with cell culture, it becomes a powerful tool for exploring cellular functions, protein expression, and drug responses. This article explores the comprehensive use of ELISA in cell cultures, including its principles, steps, variations, and applications in research and diagnostics.
What is ELISA in Cell Culture?
Key Components of ELISA in Cell Culture
- Cell Monolayer: Cultured cells grown on plates or culture flasks.
- Target Antigen/Protein: Biomolecules of interest expressed by the cultured cells.
- Primary Antibody: Binds specifically to the target antigen.
- Secondary Antibody: Conjugated to an enzyme (e.g., HRP or AP) for signal detection.
- Substrate: Reacts with the enzyme to produce a measurable signal.
- Detection System: Optical methods such as spectrophotometry.
Principles of ELISA in Cell Culture
The principle of ELISA revolves around the specific binding between antigens and antibodies. The process involves immobilizing antigens (proteins of interest) onto a solid surface, followed by a series of binding reactions using labeled antibodies and enzymatic detection.
- Step 1: Cells are grown and treated to produce target antigens.
- Step 2: Antigens are immobilized on the surface of the well or detected in the culture supernatant.
- Step 3: Primary antibodies bind specifically to the antigen.
- Step 4: A labeled secondary antibody attaches to the primary antibody.
- Step 5: A substrate reacts with the enzyme, producing a colorimetric or chemiluminescent signal.
- Step 6: Quantification of the signal correlates with the antigen concentratio
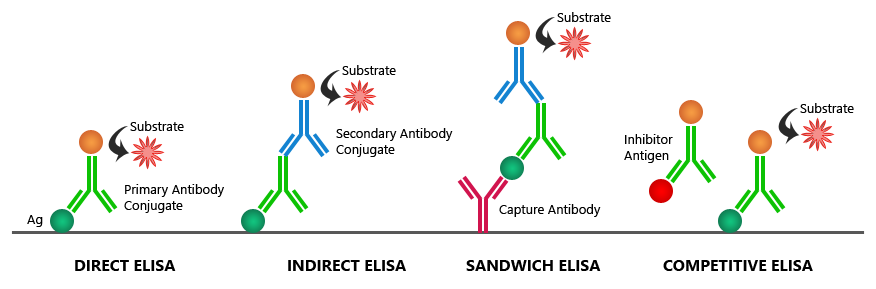
Types of ELISA Used in Cell Culture
- Direct ELISA- In direct ELISA, the target protein in the cell culture is directly immobilized onto the ELISA plate. A labeled primary antibody binds to the target, followed by signal detection.
- Advantages: Fast and simple with fewer steps.
- Limitations: Limited sensitivity due to no signal amplification.
- Indirect ELISA- Indirect ELISA involves two layers of antibodies: the primary antibody binds to the target antigen, and the enzyme-conjugated secondary antibody binds to the primary antibody.
- Advantages: Higher sensitivity due to signal amplification.
- Limitations: Longer protocol with multiple steps.
- Sandwich ELISA- In sandwich ELISA, two antibodies (capture and detection antibodies) are used to
“sandwich” the antigen. This format is particularly effective for cell culture samples containing low- abundance proteins.
- Advantages: High specificity and sensitivity.
- Applications: Cytokine detection, biomarker analysis.
- Competitive ELISA- In competitive ELISA, the sample antigen competes with a labeled antigen for binding to the capture antibody.
- Advantages: Suitable for small molecules and complex samples.
- Applications: Hormone detection, inhibitor studies
Steps to Perform ELISA on Cell Cultures
- Cell Culture Preparation: Grow the cells in a suitable medium under controlled conditions.
- Stimulation and Treatment: Treat the cells to induce the production of target antigens or cellular responses.
- Cell Lysis or Supernatant Collection: Harvest cell lysates or supernatants depending on the assay type.
- Plate Coating: Coat the ELISA plate with cell lysates or capture antibodies.
- Blocking: Block non-specific binding sites with a blocking buffer (e.g., BSA).
- Primary Antibody Incubation: Add the primary antibody specific to the target antigen.
- Secondary Antibody Addition: Apply the enzyme-conjugated secondary antibody.
- Signal Detection: Add the substrate solution and measure the optical density using a spectrophotometer.
- Data Analysis: Correlate the optical density values with the target antigen concentration using standard curves.
Applications of ELISA in Cell Culture
- Quantification of Cytokines and Growth Factors – ELISA is commonly used to detect and quantify cytokines, such as IL-6, TNF-α, and growth factors released in cell culture supernatants. This information is essential for studying cellular responses to stimuli or drugs.
- Protein Expression Analysis – Researchers use ELISA to measure protein expression levels in cultured cells after genetic modifications, drug treatments, or environmental changes.
- Monitoring Cell Signaling Pathways – ELISA helps identify and quantify key signaling molecules, such as phosphorylated proteins, involved in critical cellular pathways like MAPK, PI3K-AKT, and JAK-STAT.
- Biomarker Screening and Drug Discovery – In drug discovery, ELISA plays a pivotal role in detecting biomarkers for disease progression and evaluating the efficacy of new drug candidates in cell cultures.
Advantages of ELISA in Cell Culture
- High Sensitivity: Detects low concentrations of target molecules.
- Specificity: High specificity due to antigen-antibody interactions.
- Quantitative Results: Provides accurate quantification of biomolecules.
- Versatility: Suitable for detecting proteins, peptides, and small molecules.
- Scalability: Ideal for high-throughput screening in drug discovery and research.
Challenges and Considerations
While ELISA is highly effective, certain challenges must be addressed for optimal results:
- Sample Preparation: Proper cell lysis and supernatant collection are critical.
- Background Noise: Use appropriate blocking buffers to reduce non-specific binding.
- Antibody Quality: Ensure high-quality primary and secondary antibodies for specificity.
- Standard Curves: Use reliable standard curves for accurate quantification.
Conclusion
The Enzyme-Linked Immunosorbent Assay (ELISA) remains a cornerstone technique in cell culture research, offering unparalleled sensitivity and accuracy in detecting and quantifying cellular proteins, cytokines, and biomarkers. With its various formats, ELISA provides versatile applications in cell biology, drug discovery, and diagnostics. By addressing critical factors like sample preparation, antibody quality, and proper execution, ELISA continues to drive advancements in both basic and applied biological sciences.

